ISO 13485 was published by an international organization for standards. It defines the standards and requirements of the quality management system that can be used by any organization that anything with to do is design and development, provision and support of medical devices in any of the stages of the life cycle.
We are rendering ISO 13485:2016 certification in Singapore to our most valuable clients in the best way. We are achieving a higher position in this field by our constant dedication and determination. This standard is specifically for the medical devices industry. If you are a manufacturer, supplier, and traders of medical devices, you are eligible to get this ISO 13485:2016 Certification.
For more information call us now at anywhere, anytime.
BENEFITS OF ISO 13485:2016 Certification:
- Improve customer satisfaction
- Improved performance
- Higher Prestige
- A User-Centered Approach To Product Development.
- Reduces operational cost
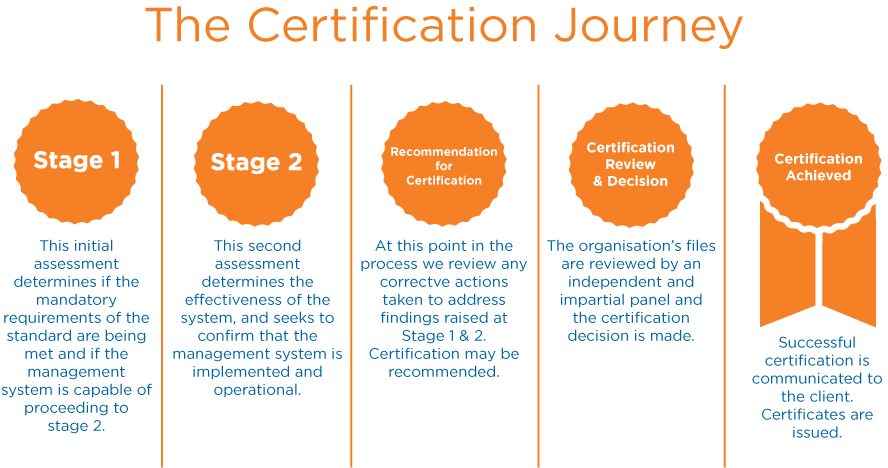
How’s about my Certification Journey? It’s a simple way…..